The Efficacy and Safety of Bedaquiline in the Treatment of Pulmonary Tuberculosis Patients: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
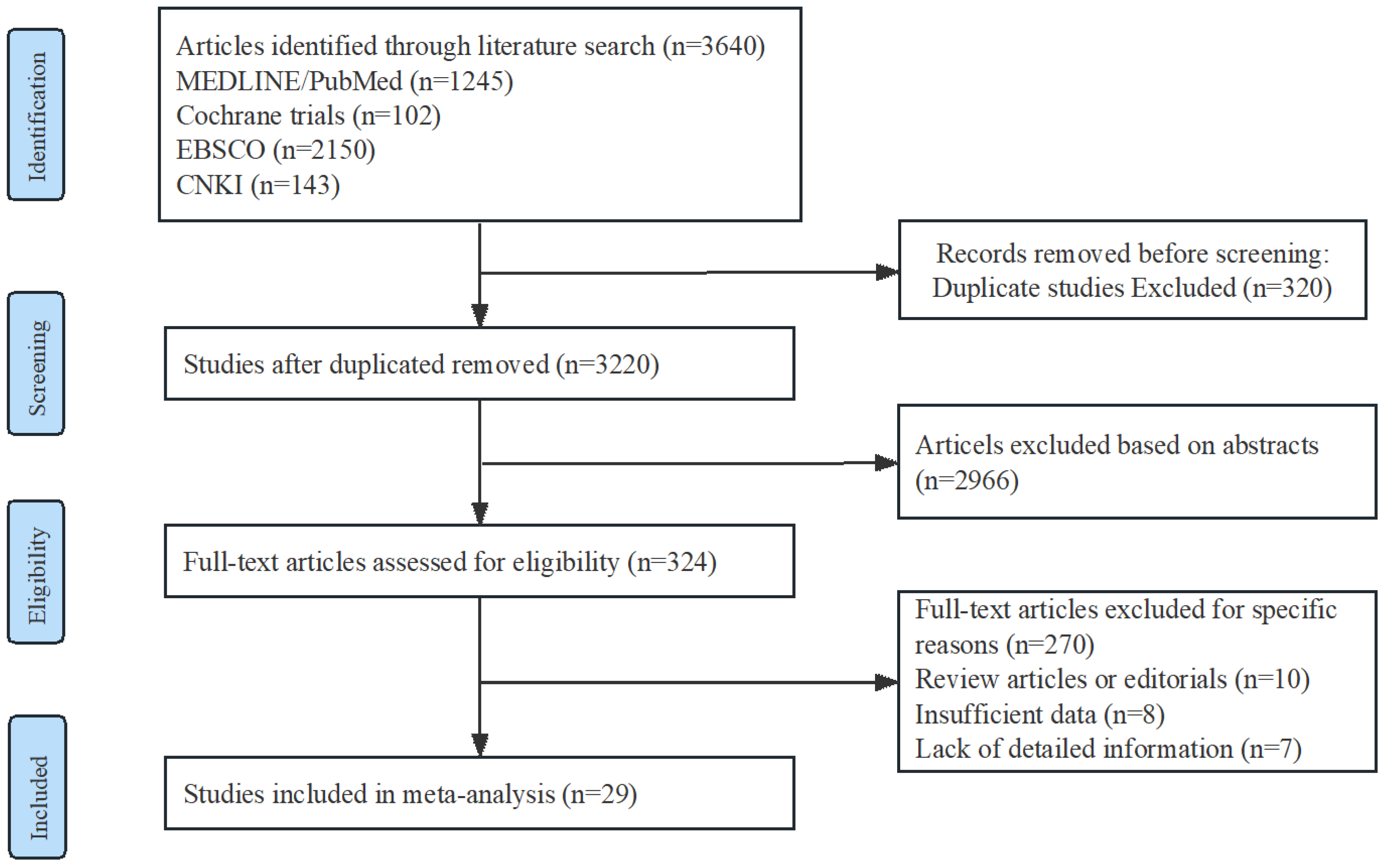
2.1. Study Selection and Characteristics
| First Author and Study Year | Study Design | Recruitment Date | Setting | Population | Sample Size | Regimens and Arms Used | Duration of Treatment |
|---|---|---|---|---|---|---|---|
| Diacon et al., 2009 [10] | RCT | June 2007–January 2008 | A hospital, South Africa | Adults, MDR-TB, treatment-naive, pulmonary sputum smear-positive; Excl. HIV-positive on ART or CD4 < 300 cells/μL. | 44 | Arm 1: BR + Bdq Arm 2: BR + placebo | 8 weeks |
| Diacon et al., 2012 [11] | RCT | N. S | A hospital, South Africa | Adults, MDR-TB; pulmonary sputum smear-positive; Excl. HIV-positive on ART or CD4 < 300 cells/μL. | 44 | Arm 1: BR + Bdq Arm 2: BR + placebo | 104 weeks |
| Diacon et al., 2012 [12] | RCT | October 2010–August 2011 | A hospital, South Africa | Adults, treatment-naive, pulmonary Rs-TB; Excl. HIV-positive on ART or CD4 ≤ 300 cells/μL. | 85 | Arm 1: Pa + Bdq Arm 2: Bdq Arm 3: Bdq + Z Arm 4: Pa + Z Arm 5: Pa + Mfx + Z Arm 6: HRZE | 2 weeks |
| Diacon et al., 2014 [13] | RCT | June 2007–March 2010 | Brazil, India, Latvia, Peru, the Philippines, Russia, South Africa, Thailand | Adults, MDR-TB, treatment-naive, pulmonary sputum smear-positive; Excl. HIV-positive on CD4 < 300 cells/μL. | 160 | Arm 1: BR + Bdq Arm 2: BR + placebo | 120 weeks |
| Diacon et al., 2015 [14] | RCT | October 2012–April 2013 | Outpatient clinics in Cape Town, South Africa | Adults, treatment-naive, pulmonary sputum smear-positive Rs and Hs-TB; Excl. HIV-positive with CD4 ≤ 300 cells/μL. | 105 | Arm 1: Bdq + Pa + Z + C Arm 2: Bdq + Pa + Z Arm 3: Bdq + Pa + C Arm 4: Bdq + Z + C Arm 5: Z Arm 6:C Arm 7: HRZE | 2 weeks |
| Esmail et al., 2022 [15] | RCT | November 2015–December 2020 | Five centres, South Africa | Adults, treatment-naive, MDR/RR-TB, pulmonary sputum smear-positive; Excl. XDR-TB, pre-XDR-TB. | 111 | Arm 1: BR + Bdq Arm 2: BR | 96 weeks |
| Ling et al., 2021 [16] | RCT | September 2018–January 2020 | A hospital, China | Adults, MDR-TB; Excl. HIV-positive. | 64 | Arm 1: BR + Bdq Arm 2: BR | 24 weeks |
| Mou. 2021 [17] | RCT | February 2019–May 2020 | A hospital, China | Adults, MDR-TB. | 63 | Arm 1: BR + Bdq Arm 2: BR + placebo | 72 weeks |
| Ren et al., 2021 [18] | RCT | December 2017–June 2019 | A hospital, China | Adults, MDR-TB; Excl. HIV-positive, HBV-positive. | 60 | Arm 1: BR + Bdq Arm 2: BR | 24 weeks |
| Tweed et al., 2019 [19] | RCT with single Rr-TB arm | October 2014–February 2016 | South Africa, Tanzania, Uganda | Adults, RR-TB; treatment-naive pulmonary, sputum smear-positive Rs-TB; Excl. HIV-positive with CD4 < 200 cells/mL. | 240 | Arm 1: Bdq + Pa + Z Arm 2: Bdq 200 + Pa + Z Control: HRZE | 8 weeks |
| Wang 2019 [20] | RCT | March 2019–August 2020 | A hospital, China | Adults, MDR-TB. | 69 | Arm 1: BR + Bdq Arm 2: BR | 26 weeks |
| Wu 2020 [21] | RCT | January 2018–July 2018 | A hospital, China | Adults, MDR-TB; sputum smear-positive. | 34 | Arm 1: BR + Bdq Arm 2: BR | 24 weeks |
| Fu et al., 2021 [22] | PCS | April 2019–August 2020 | A hospital, China | DR/MDR-TB Excl. HIV-positive, AIDS. | 103 | Arm 1: BR + Bdq Arm 2: BR | 16 weeks |
| Chang et al., 2021 [23] | PCS | January 2018–June 2019 | A hospital, China | Adults, MDR-TB. | 90 | Arm 1: BR + Bdq Arm 2: BR | 24 weeks |
| Kempker et al., 2020 [24] | PCS | December 2015–May 2017 | A centre, Georgia, USA | Adults, Smear positive MDR-TB. | 95 | Arm 1: BR + Bdq Arm 2: BR + DLM | 24 weeks |
| Kim et al., 2018 [25] | PCS | January 2015–October 2017 | Hospitals, South Korea | Adults, MDR-TB. | 50 | Arm 1: BR + Bdq Arm 2: BR + DLM | 24 weeks |
| Olayanju et al., 2018 [26] | PCS | January 2008–June 2017 | A hospital, South Africa | Adults, XDR-TB. | 272 | Arm 1: BR + Bdq Arm 2: BR | 96 weeks |
| Chen et al., 2021 [27] | RCS | June 2016–August 2019 | A centre, China | Adults, RR/MDR-TB. | 112 | Arm 1: BR + Bdq Arm 2: BR | 24 weeks |
| Chesov et al., 2021 [28] | RCS | 2016–2018 | the Republic of Moldova | Adults, sputum smear-positive MDR-TB. | 228 | Arm 1: BR + Bdq Arm 2: BR | 24 weeks |
| Bastard et al., 2018 [29] | RCS | September 2005-April 2015 | Armenia | Adults, MDR-TB | 140 | Arm 1: BR + Bdq Arm 2: BR | 24 weeks |
| Kang et al., 2020 [30] | RCS | September 2016–February 2018; | A centre, South Korea | MDR-TB | 215 | Arm 1: BR + Bdq Arm 2: BR + DLM | d48 weeks |
| Liu et al., 2021 [31] | RCS | January 2017–January 2018 | A hospital, China | Adults, RR/MDR-TB | 174 | Arm 1:BR + Bdq Arm 2:BR | 96 weeks |
| Padayatchi et al., 2020 [32] | RCS | January 2014–November 2015 | A hospital, South Africa | Adults, DR-TB | 256 | Arm 1: BR + Bdq Arm2: BR | 24 weeks |
| Ren et al., 2021 [33] | RCS | June 2018–January 2020 | A hospital, China | Adults, MDR-TB | 60 | Arm 1: BR + Bdq Arm 2: BR | 24 weeks |
| Schnippel et al., 2018 [34] | RCS | July 2014–March 2016; | EDRweb, South Africa | Adults, DR-TB | 19,617 | Arm 1: BR + Bdq Arm 2: BR + SLID | 72 weeks |
| Taune et al., 2019 [35] | RCS | June 2015–December 2017 | Papua New Guinea | Adults, RR/MDR-TB | 277 | Arm 1: BR + Bdq Arm 2: BR | 24 weeks |
| Zhang et al., 2022 [36] | RCS | November 2018–December 2020 | A centre, China | Adults, RR/MDR-TB | 127 | Arm 1: BR + Bdq Arm 2: BR | 24 weeks |
| Zhao et al., 2019 [37] | RCS | October 2014–October 2016 | EDRweb, South Africa | Adults, MDR-TB Excl. pre-XDR-TB, XDR-TB | 330 | Arm 1: BR + Bdq Arm 2: BR | 48 weeks |
| Hwang et al., 2021 [38] | RCS | September 2016–February 2018 | A hospital, South Africa | Adults, MDR-TB | 260 | Arm 1: BR + Bdq Arm 2: BR + DLM | 24 weeks |
2.2. Risk of Bias
2.3. Efficacy of Interventions
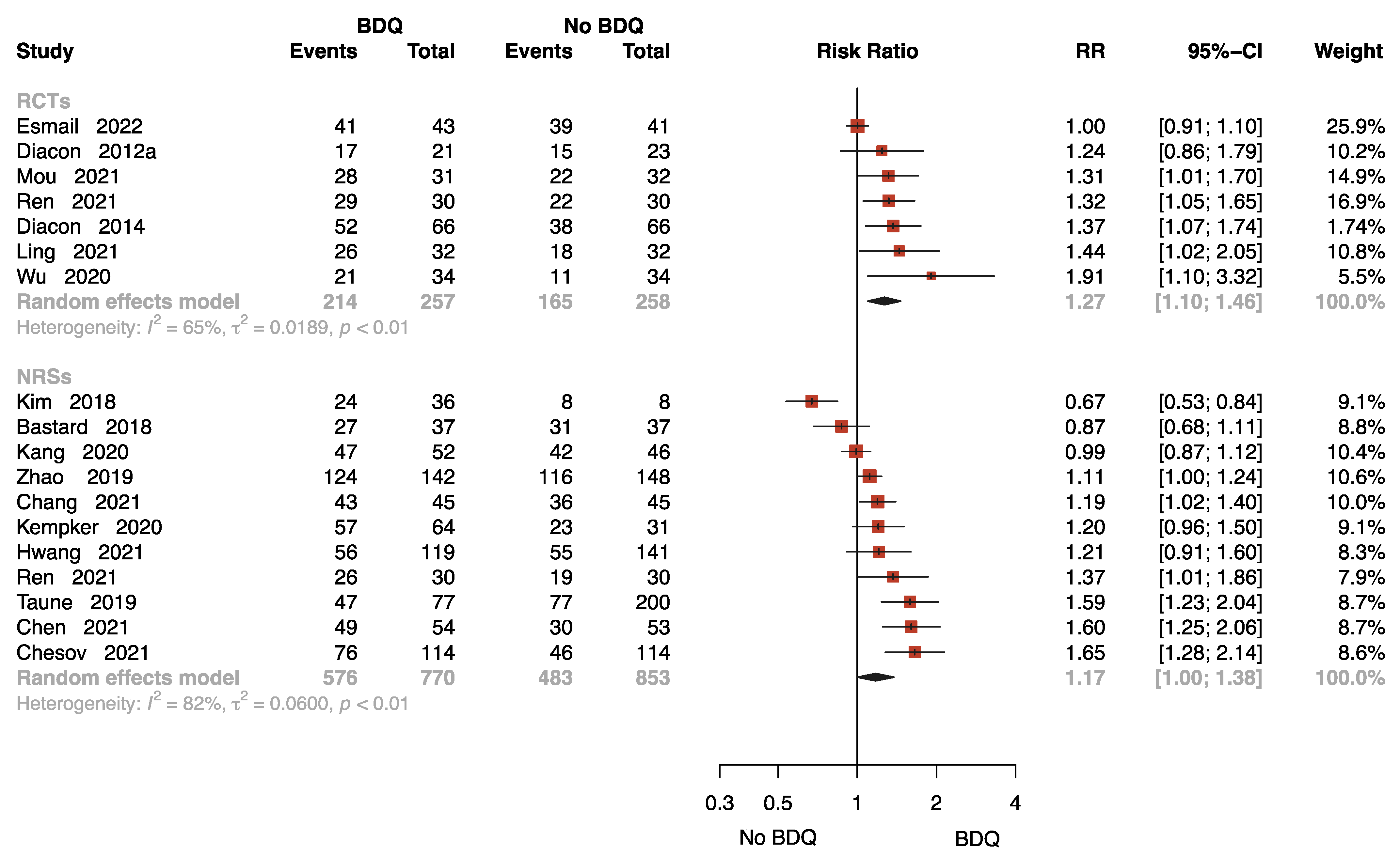
2.3.1. The Rate of Sputum Culture Conversion
The Rate of Sputum Culture Conversion at 8 Weeks
The Rate of Sputum Culture Conversion at 24 Weeks
The Rate of Sputum Culture Conversion with Follow-Up
2.3.2. The Other Treatment Outcomes
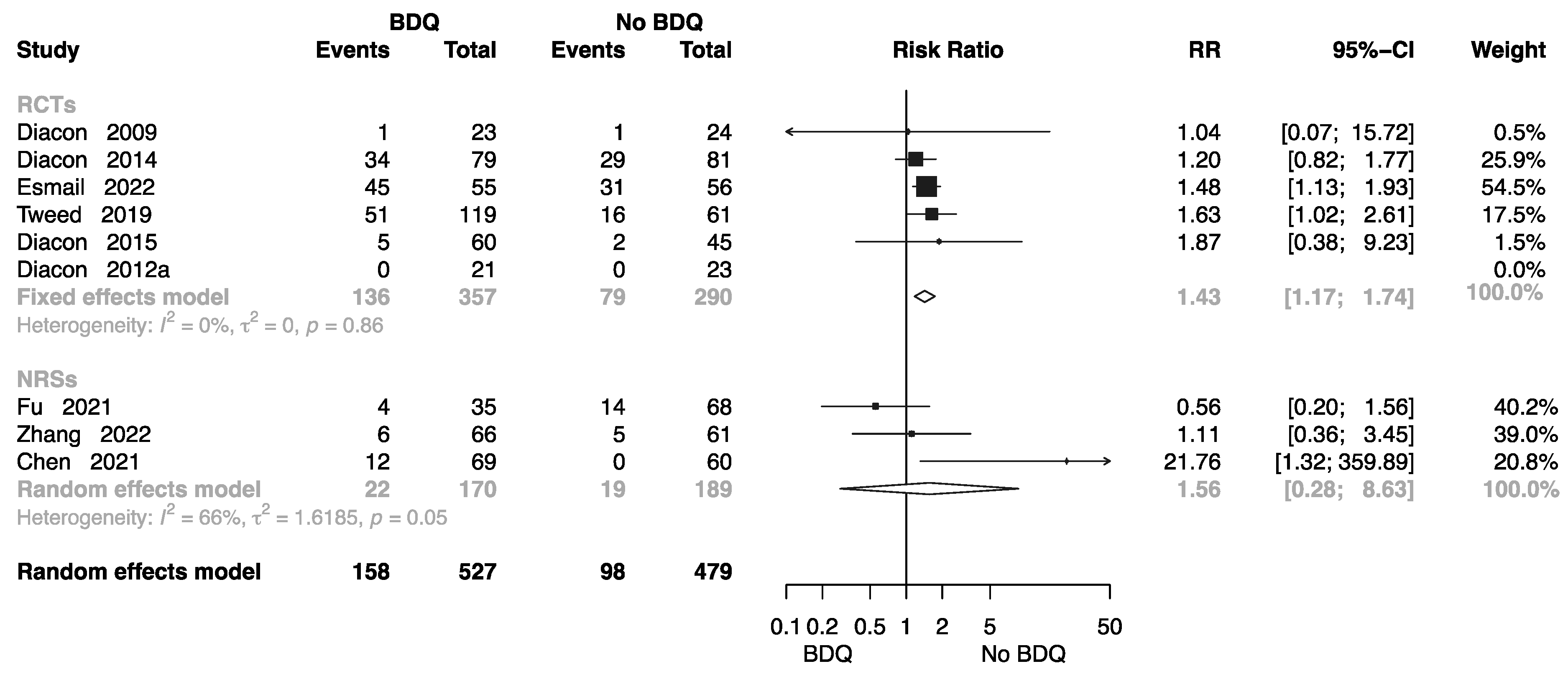
The Rate of Complete at End of the Treatment
The Rate of Cure at End of the Treatment
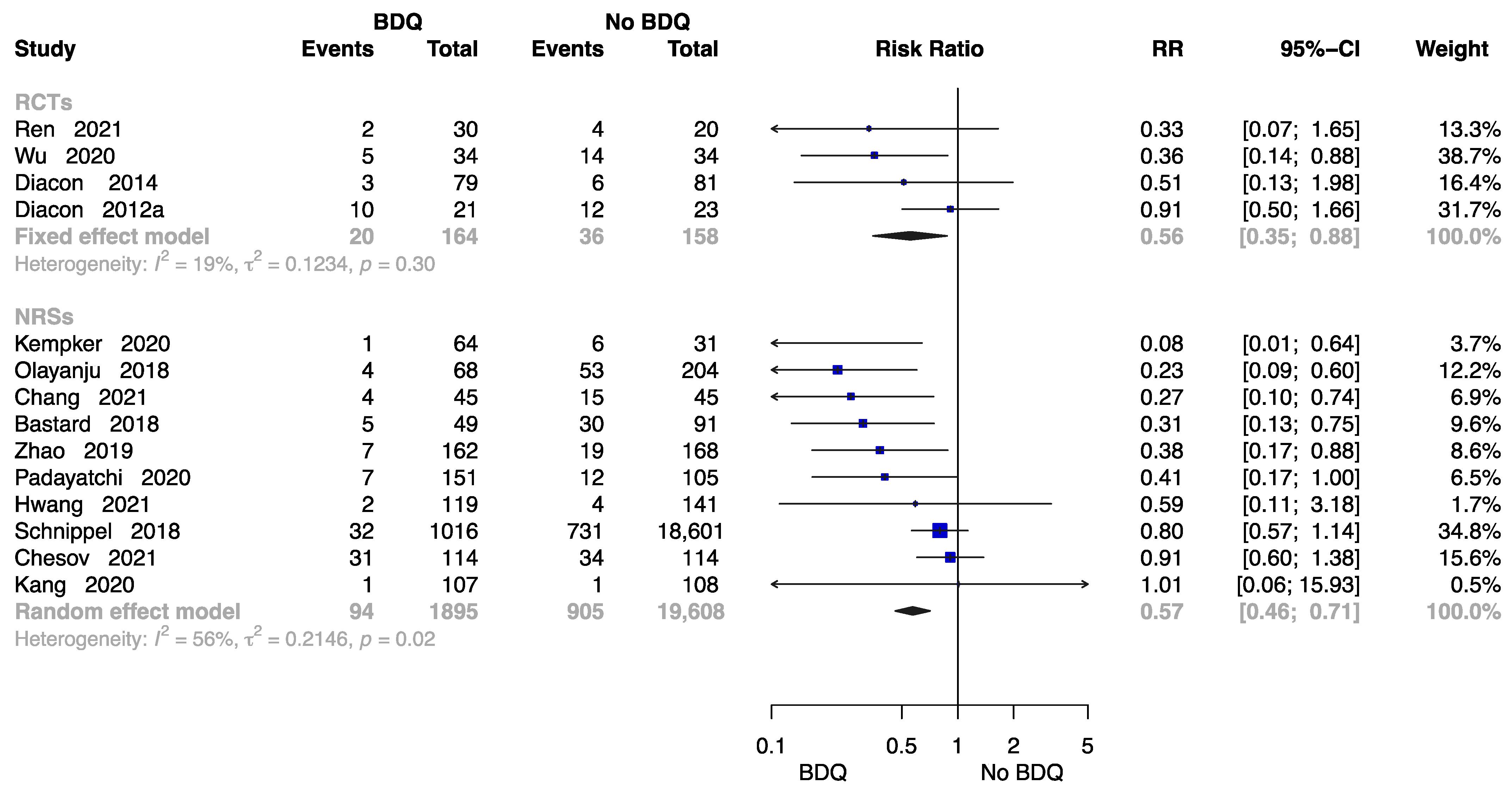
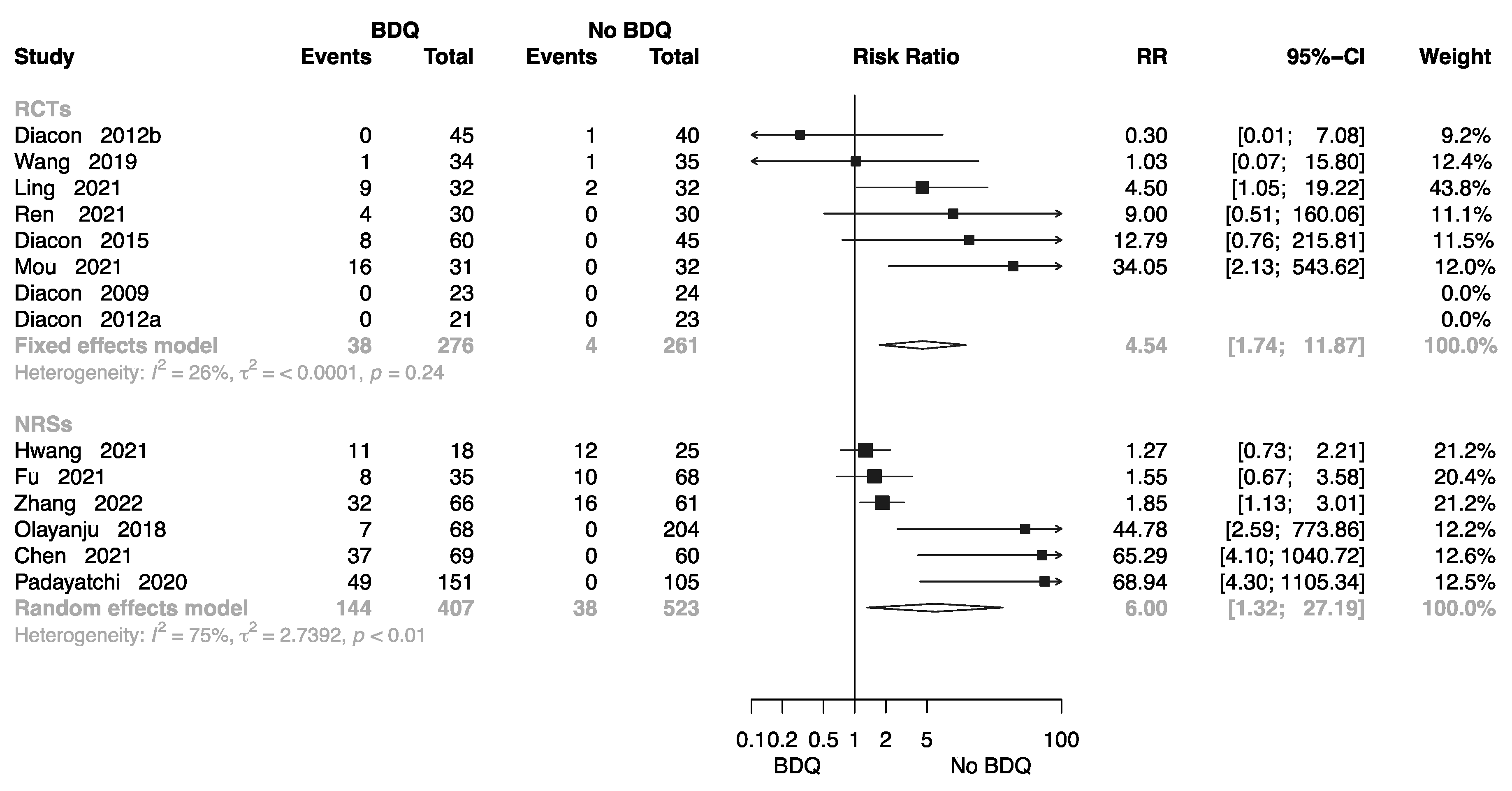
The Rate of All-Cause Death at End of the Treatment
The Failure Rate at End of the Treatment
The Rate of Lost to Follow-Up at End of the Treatment
2.4. Safety of Interventions
2.4.1. Cardiotoxicity
2.4.2. Hepatotoxicity
2.4.3. Grade 3–5 Adverse Events
2.5. Quality of Evidence Assessment
2.6. Subgroup Analysis
2.7. Sensitivity Analysis
3. Materials and Methods
3.1. Protocol Registration
3.2. Data Sources
3.3. Selection Criteria for Trials
- (1)
- (2)
- Intervention: Anti-TB treatment (ATT) regimen with BDQ or BDQ-containing regimens at any dose and for any duration.
- (3)
- Comparator: Another ATT regimen without BDQ.
- (4)
- Primary outcomes: Efficacy of BDQ-containing treatment regimen, including the rate of sputum culture conversion, cure, all-cause death, treatment failure, and lost to follow-up at the end of treatment.
- (5)
- Secondary outcome: Safety of BDQ-containing treatment regimen, including the incidence of cardiotoxicity, hepatotoxicity, and grade 3–5 adverse events during the treatment period.
- (6)
- Study design: Included RCTs and NRSs such as retrospective cohort studies (RCSs) or prospective cohort studies (PCSs).
- (7)
- Exclusion: Studies without a comparison group were excluded from the analysis.
3.4. Trial Identification
3.5. Data Extraction
3.6. Quality and Risk of Bias Assessment
3.7. Definitions
3.8. Data Synthesis and Analysis
4. Discussion
4.1. Summary of Findings
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021.
- World Health Organization. WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment; World Health Organization: Geneva, Switzerland, 2019.
- Andries, K.; Verhasselt, P.; Guillemont, J.; Göhlmann, H.W.; Neefs, J.M.; Winkler, H.; Van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005, 307, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Koul, A.; Dendouga, N.; Vergauwen, K.; Molenberghs, B.; Vranckx, L.; Willebrords, R.; Ristic, Z.; Lill, H.; Dorange, I.; Guillemont, J.; et al. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat. Chem. Biol. 2007, 3, 323–324. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Best-Practice Statement on the Off-Label Use of Bedaquiline and Delamanid for the Treatment of Multidrug-Resistant Tuberculosis; World Health Organization: Geneva, Switzerland, 2017.
- Cohen, K.; Maartens, G. A safety evaluation of bedaquiline for the treatment of multi-drug resistant tuberculosis. Expert Opin. Drug Saf. 2019, 18, 875–882. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf (accessed on 12 July 2023).
- Vanino, E.; Granozzi, B.; Akkerman, O.W.; Munoz-Torrico, M.; Palmieri, F.; Seaworth, B.; Tiberi, S.; Tadolini, M. Update of drug-resistant tuberculosis treatment guidelines: A turning point. Int. J. Infect. Dis. 2023, 130 (Suppl. 1), S12–S15. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Lists of High Burden Countries for Tuberculosis (TB), TB/HIV and Multidrug/Rifampicin-Resistant TB (MDR/RR-TB), 2021–2025: Background Document; World Health Organization: Geneva, Switzerland, 2021.
- Diacon, A.H.; Pym, A.; Grobusch, M.; Patientia, R.; Rustomjee, R.; Page-Shipp, L.; Pistorius, C.; Krause, R.; Bogoshi, M.; Churchyard, G.; et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 2009, 360, 2397–2405. [Google Scholar] [CrossRef]
- Diacon, A.H.; Donald, P.R.; Pym, A.; Grobusch, M.; Patientia, R.F.; Mahanyele, R.; Bantubani, N.; Narasimooloo, R.; De Marez, T.; van Heeswijk, R.; et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: Long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob. Agents Chemother. 2012, 56, 3271–3276. [Google Scholar] [CrossRef]
- Diacon, A.H.; Dawson, R.; von Groote-Bidlingmaier, F.; Symons, G.; Venter, A.; Donald, P.R.; van Niekerk, C.; Everitt, D.; Winter, H.; Becker, P.; et al. 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: A randomised trial. Lancet 2012, 380, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Diacon, A.H.; Pym, A.; Grobusch, M.P.; de los Rios, J.M.; Gotuzzo, E.; Vasilyeva, I.; Leimane, V.; Andries, K.; Bakare, N.; De Marez, T.; et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N. Engl. J. Med. 2014, 371, 723–732. [Google Scholar] [CrossRef]
- Diacon, A.H.; Dawson, R.; von Groote-Bidlingmaier, F.; Symons, G.; Venter, A.; Donald, P.R.; van Niekerk, C.; Everitt, D.; Hutchings, J.; Burger, D.A.; et al. Bactericidal activity of pyrazinamide and clofazimine alone and in combinations with pretomanid and bedaquiline. Am. J. Respir. Crit. Care Med. 2015, 191, 943–953. [Google Scholar] [CrossRef]
- Esmail, A.; Oelofse, S.; Lombard, C.; Perumal, R.; Mbuthini, L.; Goolam Mahomed, A.; Variava, E.; Black, J.; Oluboyo, P.; Gwentshu, N.; et al. An All-Oral 6-Month Regimen for Multidrug-Resistant TB (the NExT Study): A Multicenter, Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2022, 24, 1024–1031. [Google Scholar]
- Ling, H.; Chen, W.; He, X. Clinical efficacy of bedaquiline fumarate tablets in the treatment of multidrug-resistant tuberculosis. J. Guangxi Med. Univ. 2021, 38, 1442–1447. [Google Scholar]
- Mou, H. The curative effects and safety of bedaquoline in treatment of multi drug resistant tuberculosis. Hebei Med. J. 2021, 43, 2955–2957+2961. [Google Scholar]
- Ren, N.; Hu, S.; Liu, J.; Chen, Y. Observation of Effect of bedaquiline fumarate tablets in treatment of multi-drug resistant tuberculosis. J. Clin. Pulm. Med. 2021, 26, 1047–1051. [Google Scholar]
- Tweed, C.D.; Dawson, R.; Burger, D.A.; Conradie, A.; Crook, A.M.; Mendel, C.M.; Conradie, F.; Diacon, A.H.; Ntinginya, N.E.; Everitt, D.E.; et al. Bedaquiline, moxifloxacin, pretomanid, and pyrazinamide during the first 8 weeks of treatment of patients with drug-susceptible or drug-resistant pulmonary tuberculosis: A multicentre, open-label, partially randomised, phase 2b trial. Lancet Respir. Med. 2019, 7, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Wang, F. The Clinical Efficacy and Safety of Bedaquiline and Moxifloxacin in the Treatment of Multi-drug Resistant Tuberculosis. Chin. Pract. J. Rural. Dr. 2021, 28, 55–57. [Google Scholar]
- Wu, H. Observe the Effect of Bedaquiline Combined with Conventional Anti-tuberculosis Drugs in the Treatment of Multi-drug Resistant Pulmonary Tuberculosis. Guide China Med. 2020, 18, 48–49. [Google Scholar]
- Fu, L.; Weng, T.; Sun, F.; Zhang, P.; Li, H.; Li, Y.; Yang, Q.; Cai, Y.; Zhang, X.; Liang, H.; et al. Insignificant difference in culture conversion between bedaquiline-containing and bedaquiline-free all-oral short regimens for multidrug-resistant tuberculosis. Int. J. Infect. Dis. 2021, 111, 138–147. [Google Scholar] [CrossRef]
- Chang, R.; Ke, C. Efficacy and safety of Bedaquiline in the treatment of multidrug-resistant tuberculosis. Clin. Medicat. J. 2021, 19, 57–60. [Google Scholar]
- Kempker, R.R.; Mikiashvili, L.; Zhao, Y.; Benkeser, D.; Barbakadze, K.; Bablishvili, N.; Avaliani, Z.; Peloquin, C.A.; Blumberg, H.M.; Kipiani, M. Clinical Outcomes among Patients with Drug-resistant Tuberculosis Receiving Bedaquiline- or Delamanid-Containing Regimens. Clin. Infect. Dis. 2020, 71, 2336–2344. [Google Scholar] [CrossRef]
- Kim, C.T.; Kim, T.O.; Shin, H.J.; Ko, Y.C.; Hun Choe, Y.; Kim, H.R.; Kwon, Y.S. Bedaquiline and delamanid for the treatment of multidrug-resistant tuberculosis: A multicentre cohort study in Korea. Eur. Respir. J. 2018, 51, 1702467. [Google Scholar] [CrossRef]
- Olayanju, O.; Limberis, J.; Esmail, A.; Oelofse, S.; Gina, P.; Pietersen, E. Long-term bedaquiline-related treatment outcomes in patients with extensively drug-resistant tuberculosis from South Africa. Eur. Respir. J. 2018, 51, 1800544. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Z.; Huang, T.; Lu, X.; Li, P.; Lin, R.; Chen, X.; Liu, Y.; Gao, M.; Wu, G.; et al. Efficacy and safety of combination regimen containing bedaquiline in the treatment of multidrug-resistant tuberculosis. West China Med. J. 2021, 36, 1392–1398. [Google Scholar]
- Chesov, D.; Heyckendorf, J.; Alexandru, S.; Donica, A.; Chesov, E.; Reimann, M.; Crudu, V.; Botnaru, V.; Lange, C. Impact of bedaquiline on treatment outcomes of multidrug-resistant tuberculosis in a high-burden country. Eur. Respir. J. 2021, 57, 2002544. [Google Scholar] [CrossRef]
- Bastard, M.; Guglielmetti, L.; Huerga, H.; Hayrapetyan, A.; Khachatryan, N.; Yegiazaryan, L.; Faqirzai, J.; Hovhannisyan, L.; Varaine, F.; Hewison, C. Bedaquiline and Repurposed Drugs for Fluoroquinolone-Resistant Multidrug-Resistant Tuberculosis: How Much Better Are They? Am. J. Respir. Crit. Care Med. 2018, 198, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Jo, K.W.; Jeon, D.; Yim, J.J.; Shim, T.S. Interim treatment outcomes in multidrug-resistant tuberculosis using bedaquiline and/or delamanid in South Korea. Respir. Med. 2020, 167, 105956. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Effect of Bedaquinoline on Sputum Negative Conversion and Blood Glucose Control in Diabetic Patients with Drug-Resistant Pulmonary Tuberculosis. Med. Innov. China 2021, 18, 150–153. [Google Scholar]
- Padayatchi, N.; Bionghi, N.; Osman, F.; Naidu, N.; Ndjeka, N.; Master, I.; Brust, J.C.M.; Naidoo, K.; Ramjee, A.; O’Donnell, M. Treatment outcomes in patients with drug-resistant TB-HIV co-infection treated with bedaquiline and linezolid. Int. J. Tuberc. Lung Dis. 2020, 24, 1024–1031. [Google Scholar] [CrossRef]
- Ren, P.; Chen, Y.; Ma, Z.; Wang, J. Long term efficacy and adverse reactions of combination therapy with bedaquiline in the treatment of patients with multi drug resistant tuberculosis. Clin. Res. Pract. 2021, 6, 31–33. [Google Scholar]
- Schnippel, K.; Ndjeka, N.; Maartens, G.; Meintjes, G.; Master, I.; Ismail, N.; Hughes, J.; Ferreira, H.; Padanilam, X.; Romero, R.; et al. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 699–706. [Google Scholar] [CrossRef]
- Taune, M.; Ustero, P.; Hiashiri, S.; Huang, K.; Aia, P.; Morris, L.; Main, S.; Chan, G.; du Cros, P.; Majumdar, S.S. Successful implementation of bedaquiline for multidrug-resistant TB treatment in remote Papua New Guinea. Public Health Action 2019, 9, S73–S79. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.; Chang, T.; Liu, F. Analysis of adverse reactions of bedaquiline containing regimen in the treatment of drug-resistant pulmonary tuberculosis. Chin. J. Antituberc. 2022, 44, 239–245. [Google Scholar]
- Zhao, Y.; Fox, T.; Manning, K.; Stewart, A.; Tiffin, N.; Khomo, N.; Leslie, J.; Boulle, A.; Mudaly, V.; Kock, Y.; et al. Improved Treatment Outcomes with Bedaquiline When Substituted for Second-line Injectable Agents in Multidrug-resistant Tuberculosis: A Retrospective Cohort Study. Clin. Infect. Dis. 2019, 68, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Kang, H.; Kwon, Y.S.; Jeon, D.; Shim, T.S.; Yim, J.J. Outcomes of Multidrug-Resistant Tuberculosis Treated with Bedaquiline or Delamanid. Clin. Infect. Dis. 2021, 73, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Mase, S.; Chorba, T.; Lobue, P.; Castro, K. Provisional CDC guidelines for the use and safety monitoring of bedaquiline fumarate (Sirturo) for the treatment of multidrug-resistant tuberculosis. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2013, 62, 1–12. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Reported Tuberculosis in the United States, 2011; US Department of Health and Human Services, CDC; Atlanta, GA, USA, 2012.
- Conradie, F.; Meintjes, G.; Hughes, J.; Maartens, G.; Ferreira, H.; Siwendu, S.; Master, I.; Ndjeka, N. Clinical access to Bedaquiline Programme for the treatment of drug-resistant tuberculosis. S. Afr. Med. J. 2014, 104, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Schiavo, J.H. PROSPERO: An international register of systematic review protocols. Med. Ref. Serv. Q. 2019, 38, 171–180. [Google Scholar] [CrossRef]
- World Health Organization. Policy Framework for Implementing New Tuberculosis Diagnostics; WHO: Geneva, Switzerland, 2010.
- World Health Organization. Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB; World Health Organization: Geneva, Switzerland, 2013.
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G.; Group, C.S.M. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley Online Library: Hoboken, NJ, USA; Available online: www.training.cochrane.org/handbook (accessed on 28 August 2023).
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definitions and Reporting Framework for Tuberculosis–2013 Revision: Updated December 2014 and January 2020; World Health Organization: Geneva, Switzerland, 2013.
- Gasparyan, S.B.; Folkvaljon, F.; Bengtsson, O.; Buenconsejo, J.; Koch, G.G. Adjusted win ratio with stratification: Calculation methods and interpretation. Stat. Methods Med. Res. 2021, 30, 580–611. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team RStudio Integrated Development for R. Available online: https://www.rstudio.com/ (accessed on 28 August 2023).
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Fixed effect and random effects meta-analysis. In Meta-Analysis with R; Springer: Berlin/Heidelberg, Germany, 2015; pp. 21–53. [Google Scholar]
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime. 2022. Available online: gradepro.org (accessed on 28 August 2023).
- Worley, M.V.; Estrada, S.J. Bedaquiline: A novel antitubercular agent for the treatment of multidrug-resistant tuberculosis. Pharmacotherapy 2014, 34, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Hards, K.; Robson, J.R.; Berney, M.; Shaw, L.; Bald, D.; Koul, A.; Andries, K.; Cook, G.M. Bactericidal mode of action of bedaquiline. J. Antimicrob. Chemother. 2015, 70, 2028–2037. [Google Scholar] [CrossRef]
- Khoshnood, S.; Goudarzi, M.; Taki, E.; Darbandi, A.; Kouhsari, E.; Heidary, M.; Motahar, M.; Moradi, M.; Bazyar, H. Bedaquiline: Current status and future perspectives. J. Glob. Antimicrob. Resist. 2021, 25, 48–59. [Google Scholar] [CrossRef]
- Pontali, E.; Sotgiu, G.; D’Ambrosio, L.; Centis, R.; Migliori, G.B. Bedaquiline and multidrug-resistant tuberculosis: A systematic and critical analysis of the evidence. Eur. Respir. J. 2016, 47, 394–402. [Google Scholar] [CrossRef]
- World Health Organization. Report of the Guideline Development Group Meeting on the Use of Bedaquiline in the Treatment of Multidrug-Resistant Tuberculosis: A Review of Available Evidence (2016), 28–29 June 2016, Geneva, Switzerland; World Health Organization: Geneva, Switzerland, 2017.
- Mbuagbaw, L. Review of available evidence on the use of bedaquiline for the treatment of multidrug-resistant tuberculosis: Data analysis report. Organ. TWH. March 2017, 8, 6. [Google Scholar]
- Lester, R.M.; Paglialunga, S.; Johnson, I.A. QT Assessment in Early Drug Development: The Long and the Short of It. Int. J. Mol. Sci. 2019, 20, 1324. [Google Scholar] [CrossRef]
- Borisov, S.E.; Dheda, K.; Enwerem, M.; Leyet, R.R.; D’Ambrosio, L.; Centis, R.; Sotgiu, G.; Tiberi, S.; Alffenaar, J.-W.; Maryandyshev, A.; et al. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: A multicentre study. Eur. Respir. J. 2017, 49, 1700387. [Google Scholar] [CrossRef]
- Gao, M.; Gao, J.; Xie, L.; Wu, G.; Chen, W.; Chen, Y.; Pei, Y.; Li, G.; Liu, Y.; Shu, W.; et al. Early outcome and safety of bedaquiline-containing regimens for treatment of MDR- and XDR-TB in China: A multicentre study. Clin. Microbiol. Infect. 2020, 27, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, L.; Tiberi, S.; Burman, M.; Kunst, H.; Wejse, C.; Togonidze, T.; Bothamley, G.; Lange, C. QT prolongation and cardiac toxicity of new tuberculosis drugs in Europe: A Tuberculosis Network European Trialsgroup (TBnet) study. Eur. Respir. J. 2018, 52, 1800537. [Google Scholar] [CrossRef] [PubMed]
- Sandgren, A.; Vonk Noordegraaf-Schouten, J.M.; Oordt-Speets, A.M.; van Kessel, G.B.; de Vlas, S.J.; van der Werf, M.J. Identifying components for programmatic latent tuberculosis infection control in the European Union. Eurosurveillance 2016, 21, 30325. [Google Scholar] [CrossRef] [PubMed]
- Hatami, H.; Sotgiu, G.; Bostanghadiri, N.; Abadi, S.S.D.; Mesgarpour, B.; Goudarzi, H.; Migliori, G.B.; Nasiri, M.J. Bedaquiline-containing regimens and multidrug-resistant tuberculosis: A systematic review and meta-analysis. J. Bras. Pneumol. 2022, 48, e20210384. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.G.; Wu, S.Q.; He, J.Q. Efficacy of bedaquiline in the treatment of drug-resistant tuberculosis: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 970. [Google Scholar] [CrossRef]
- Bothwell, L.E.; Greene, J.A.; Podolsky, S.H.; Jones, D.S. Assessing the Gold Standard—Lessons from the History of RCTs. N. Engl. J. Med. 2016, 374, 2175–2181. [Google Scholar] [CrossRef]
- van de Berg, S.E.J.; Pelzer, P.T.; van der Land, A.J.; Abdrakhmanova, E.; Ozi, A.M.; Arias, M.; Cook-Scalise, S.; Dravniece, G.; Gebhard, A.; Juneja, S.; et al. Acceptability, feasibility, and likelihood of stakeholders implementing the novel BPaL regimen to treat extensively drug-resistant tuberculosis patients. BMC Public Health 2021, 21, 1404. [Google Scholar] [CrossRef]
- Akkerman, O.W.; Tiberi, S.; Alffenaar, J.W. Shortening MDR-TB treatment: Is treating more patients with fewer drugs better? Int. J. Tuberc. Lung Dis. 2021, 25, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, X.; Ni, H. Small studies may overestimate the effect sizes in critical care meta-analyses: A meta-epidemiological study. Crit. Care 2013, 17, R2. [Google Scholar] [CrossRef]
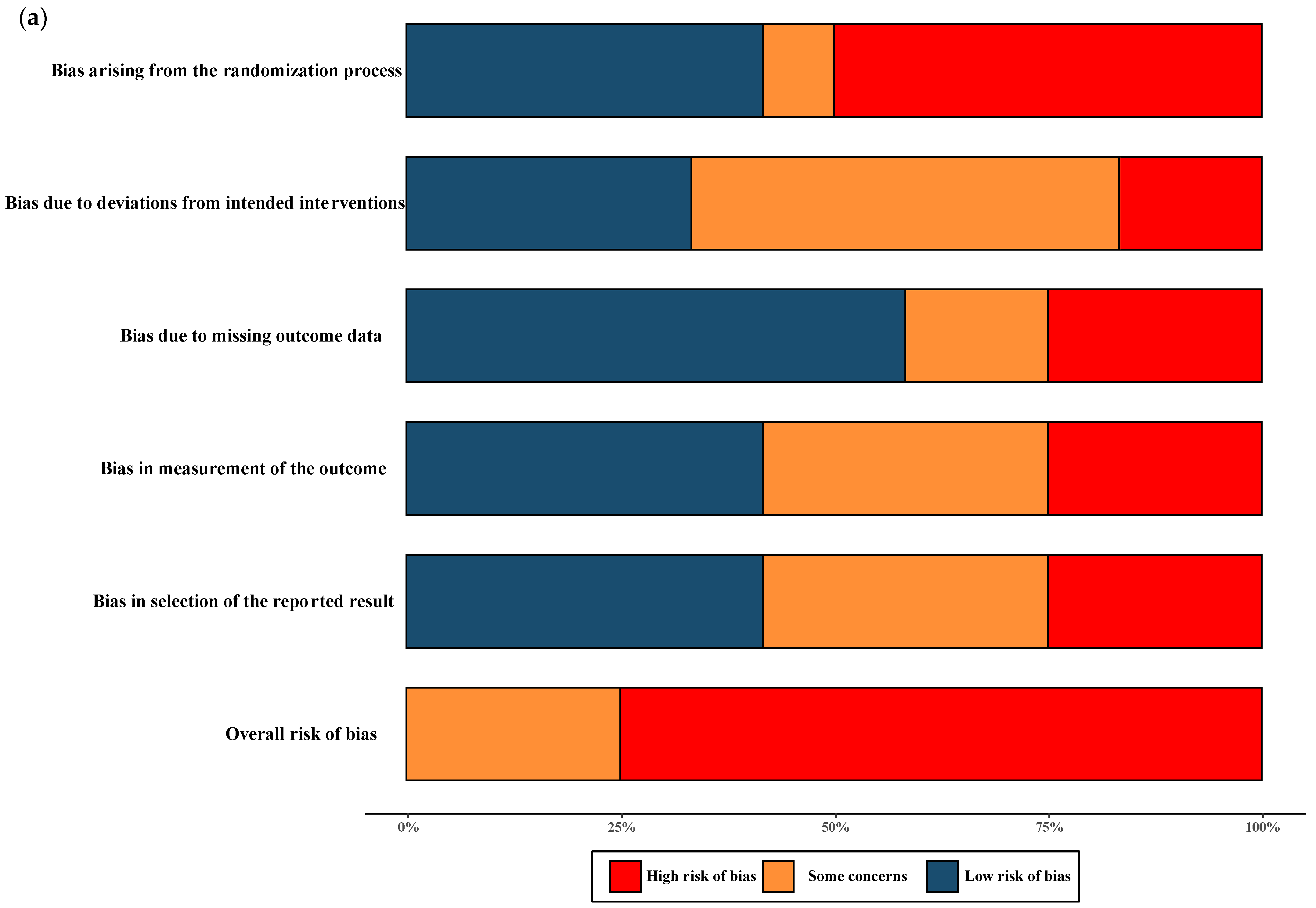




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, E.; Wu, Q.; Chen, Y.; Liu, Z.; Zhang, M.; Zhu, Y.; Wu, K.; Pan, J.; Jiang, J. The Efficacy and Safety of Bedaquiline in the Treatment of Pulmonary Tuberculosis Patients: A Systematic Review and Meta-Analysis. Antibiotics 2023, 12, 1389. https://doi.org/10.3390/antibiotics12091389
Tong E, Wu Q, Chen Y, Liu Z, Zhang M, Zhu Y, Wu K, Pan J, Jiang J. The Efficacy and Safety of Bedaquiline in the Treatment of Pulmonary Tuberculosis Patients: A Systematic Review and Meta-Analysis. Antibiotics. 2023; 12(9):1389. https://doi.org/10.3390/antibiotics12091389
Chicago/Turabian StyleTong, Enyu, Qian Wu, Yiming Chen, Zhengwei Liu, Mingwu Zhang, Yelei Zhu, Kunyang Wu, Junhang Pan, and Jianmin Jiang. 2023. "The Efficacy and Safety of Bedaquiline in the Treatment of Pulmonary Tuberculosis Patients: A Systematic Review and Meta-Analysis" Antibiotics 12, no. 9: 1389. https://doi.org/10.3390/antibiotics12091389





